Precision Over Pain: How Spinally Is Reinventing Spinal Cord Stimulation
Despite decades of clinical use, spinal cord stimulation still depends on bulky, invasive systems and outdated electrode designs. Chronic pain and motor impairment continue to affect millions — yet innovation in how we deliver neuromodulation to the spine has stalled. The intrathecal sector, which refers to products utilising the fluid-filled space directly surrounding the spinal cord, remains a largely untapped route for diagnostics, monitoring, and neuromodulation — but may offer a pathway to higher precision, clearer recordings, reduced power use, and fewer surgical risks.
At the center of a push to change that is Spinally, a European start-up developing a next‑generation intrathecal neural interface that can both stimulate and record signals from within the spinal canal. By enabling closed‑loop control without open surgery, and aiming to deliver more precise, deeper stimulation, Spinally’s ambition is to do for chronic pain patients what pacemakers did for heart disease patients, before unlocking new indications in the future.
In an exclusive interview, Pawel Soluch, CEO and co‑founder of Spinally, shares his journey from neurosurgery and neuroscience research to entrepreneurship, the story behind Spinally’s first product, FuturaLead™, and how his team plans to bring minimally invasive spinal neurotechnology to the clinic.

Pawel Soluch, CEO and co-founder, representing Spinally at Life Science Intelligence (LSI) Europe in 2025.
You’ve got quite a unique background — can you tell me about your career prior to co‑founding Spinally?
My background includes clinical work in neurosurgery, neuroscience research, university lecturing and since 2008, and entrepreneurship in neurotechnology. I served as CEO of a company, NeuroDevice, which I founded in 2008, that commercialized five medical devices, neuroscience lab equipment, and developed many more prototypes. In recent years, I’ve also been involved in consulting, serving on advisory boards for neurotech start-ups, and working as a Principal at NeuroTechX Services for a while.
I believe that this blend of experience — from scientific insight to real‑world healthcare, including the systems, the patients and the doctors — gives me a well‑rounded view of how to effectively develop medical technologies. I’m also very focused on the people on my team — having walked in their shoes helps me lead and support them, even without an engineering background.
What inspired you to get into neuroscience, and later, neurotech?
It all started with an interest in biology, which led me to neuropsychology and then medicine. Along the way I realized just how much more we still need to do to truly make a difference in patients’ lives. That feeling drives me a lot — the sense that I’m needed, especially for neurological and psychiatric patients.
As always, people played a key role. My mother studied biology, and my teachers, professors, and mentors all encouraged me. Specific patients I met during my neurosurgery experience, colleagues from my early work at the Medical University of Warsaw in neurosurgery, and later my work with NeuroDevice all influenced and guided me on this path.

Spinally’s team and collaborators in the surgical room during large animal trials.
What and who inspired you to found Spinally?
Spinally was originally envisioned by an exceptional medical doctor, Carles García-Vitoria. He has not only a brilliant mind and strong clinical experience, but also a real talent for entrepreneurship and a gift for bringing people together. The company was incorporated in 2022 by Carles, Andrés Izquierdo — who brought financial expertise — and Beatriz Llamusi, who contributed her background in neuroscience and entrepreneurship. Her other start-up, the biotech ARTHEx, has been very successful.
I joined the team in 2023 when they decided to evolve from a concept into a fully structured start-up. So while I wasn’t there at incorporation, I’m a co‑founder in the sense that I led the transformation from idea into company. Richard Nash came on board a few months before me and also played a key role in shaping Spinally in those early days. It was Richard who introduced me to the team.
Before joining, I wanted to make sure the project had real potential. Neurotech has been booming in recent years, and I didn’t want to join a project without real proof. I took time to do thorough market research and scientific validation through my network in clinics, business, and academia. Aside from one professor who was slightly skeptical, all the key opinion leaders I consulted showed strong interest in the concept. After that, I was ready to apply for the CEO role.
With so much competition in the spinal cord stimulation market, how do you and the team feel about your position? How do you differentiate yourselves?
You’re absolutely right — pain management is a crowded market. And yet, opioids remain the first-line treatment, which shows how much room there still is for innovation. Both established players and new entrants are in a kind of marathon to expand and reshape this space. The vision is to replicate the success story of pacemakers, and we at Spinally believe that’s entirely possible. Our goal is to make spinal cord stimulation more effective and, from that, create new opportunities for development.
Many of the current trends actually work in our favor because spinal cord stimulation still faces critical challenges: It needs to deliver higher efficacy, support truly closed‑loop systems, and use smaller, more energy‑efficient pulse generators. When you look at the evolution of spinal cord stimulation over the past sixty years, most of the innovation has been in electronics. Electrodes are still placed in the epidural space (the cushion of fat and soft tissue that sits right outside the spinal cord’s protective layer) and haven’t changed much in form factor. Think of it this way: Companies like Precision Neuroscience, Synchron, and Neuralink don’t rely on classical electroencephalography because recording or stimulating from outside the body has major limitations. Epidural leads sit far from the actual target — the spinal cord — and between the electrode and the cord are layers of fat, blood vessels, ligaments, and dura mater. That not only reduces stimulation precision, but it also increases energy demands and limits the ability to record high‑quality signals — something that’s essential for a truly closed‑loop system.
That’s why we developed FuturaLead™, our intrathecal lead. By placing the lead into the thecal sac (the fluid-filled space directly surrounding the spinal cord) we allow for more precise and deeper stimulation, lower power consumption, and higher‑quality signal recording. It directly addresses the limitations of epidural systems. We see these gaps as opportunities to set ourselves apart.

Spinally’s device, FuturaLead™, is placed into the fluid-filled space directly surrounding the spinal cord, aiming for improved recording, stimulation, and power consumption.
What does the future hold for Spinally — especially with fundraising and trials on the horizon?
These are unpredictable times — funding cut‑offs and layoffs at the FDA and NIH may well impact the medical device field. But in reality, you never know whether these changes will ultimately be positive or negative. As CEO, my job is to leverage opportunities, manage risks, and minimize losses. For example, in January our goal was to start the trial in June, but due to the circumstances we were at risk of a five month delay. Eventually, we found a way to address the situation and keep the project on track, and as a result we were prepared to start the study even a month earlier, in May.
That said, I actually enjoy the adrenaline and the challenges. I believe in our team and our technology, and with that belief — and our expertise — I’m optimistic that the future looks bright. The coming months are especially exciting. We are in the middle of a large animal trial and the results have exceeded our expectations. When compared with conventional leads, the differences are significant: We see an 86% reduction in the current required to activate DC fibers and a 600% reduction in ECAP thresholds (the lowest level of electrical stimulation that can reliably evoke a response from a nerve). We’re also seeing higher stimulation selectivity and an electromagnetic field that covers more of the spinal cord at the same potential. These results will be presented for the first time at NANS 2026.
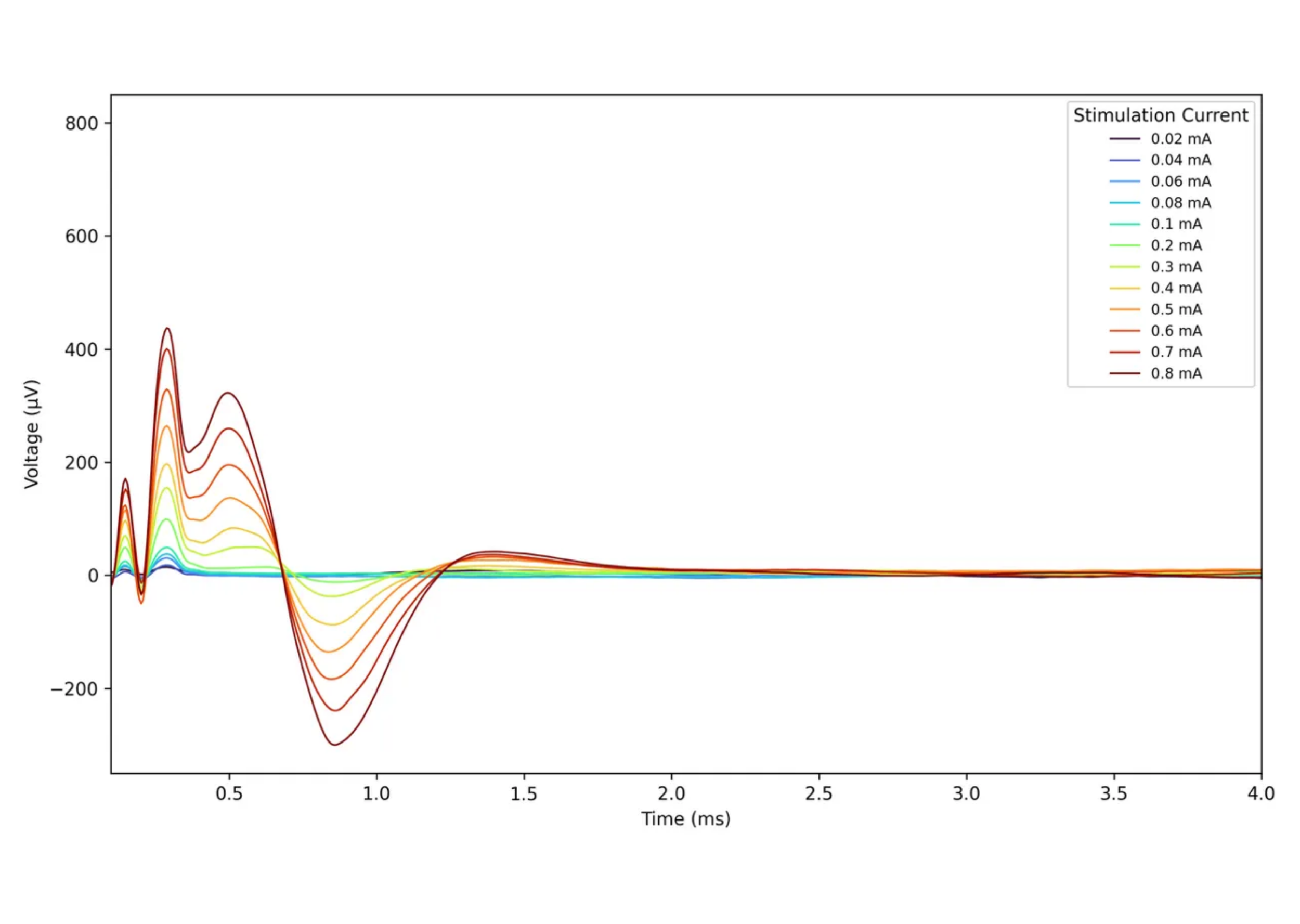
Example of intrathecal recording with FuturaLead™ usage, by Spinally.
We are also receiving early signals that market leaders have begun to show interest in our work. We launched our next financing round and, fortunately, our effort and positive pre-clinical results were rewarded, because only a few weeks after opening the round we signed a term sheet with a lead investor.
Where do you see the neurotechnology market going in the future?
I don’t like to predict, but we’d be ignorant not to recognize how much neuroprosthetics, and more broadly brain‑computer interfaces (BCIs), are evolving. Without a doubt, the future of neurotechnology will be ‘noisy’, in the best way, thanks to ongoing advancements in the BCI field. Just look at the progress over the past few years: Onward has shown promising results, Synchron is combining their product with AI assistance, and several start-ups have already obtained Investigational Device Exemptions for their products. Paradromics, Precision, Neuralink, Axoft — this is where the excitement is.
If we take a broader view of BCI, we’re also seeing innovation in the treatment of mood disorders, migraine, and neurodegenerative diseases. There’s huge potential in vagus nerve stimulation, especially in areas that are still largely untapped — like alleviating symptoms during the menstrual cycle or treating autoimmune diseases — so new companies are entering this field. We also can’t ignore that transcranial magnetic stimulation (TMS) is finally gaining market traction; recent reports project a global CAGR of around 9% through 2030, and Repetitive TMS remains the fastest‑growing segment, signaling increasing clinical adoption.
On a more personal note, I’m a bit disappointed with how slowly neuromodulation and drug‑combination therapies are developing. The potential there is huge, but we still lack strong real‑world examples of treatments that could be controlled or enhanced by neuromodulation.
Another area with great promise is next‑generation prosthetics — achieving precise control of a prosthetic arm or leg. I believe we’ll start seeing competitive demonstrations of this technology, though slowly. I’m not naive — to bring a medical technology to life takes a time, but I also believe neurotechnology is finally moving at the right pace. It’s only going to grow from here. That’s something I wish for myself, for you, for all of us.
Also published on Medium via NeuroTechX
Looking to Build a World-Class Neurotech Team?
Carter Sciences delivers personalized talent strategies backed by 20 years of international headhunting experience. We support growth-stage startups with tailored solutions and cost-effective fee structures, so you can scale without impacting your runway.
Specialisms include: Neurotechnology, Neuromodulation/Stimulation, Brain-Computer Interfaces, Wearable Devices, Neurosurgical Technology, and Private Equity/Venture Capital.
Contact Carter Sciences
